| Sample from a lake (4x time lapse) | Successfully cultivated Cymatopleura elliptica (5x time lapse) |
Creating cultures and care
Culturing diatoms
As my interest is above all the locomotion of the diatoms, I limit myself to cultivating benthic pennate (bilaterally symmetric) diatoms with few exceptions.
At the beginning of a culturing process is the collection of samples. Fortunately you can find diatoms in practically all waters. It is sufficient to place a small stone or parts of a plant in a sample container with water and to examine them later at home. For this purpose, the sample can be kept in a petri dish filled with water from the place where the sample was taken. The diatoms are carefully brushed from the sample with a soft brush.
Not only diatoms will be rinsed into the petri dish, but in addition flagellates, ciliates, rotifers, amoebae or species of single-celled algae. Sand grains, particles of the sample and organic detritus are also found in the petri dish. After a short time, the diatoms are sedimented and easily recognizable. An impression of a rich sample from a nearby small lake is given in the top left video.
It is advisable to first examine the sample at low magnification. For this purpose an inverted microscope or stereo microscope can be used. Diatoms, which one would like to cultivate, are removed with a pipette from the bottom of the petri dish and transferred into a prepared culture vessel with a nutrient solution, such as a petri dish or culture tube. If the sample contains other organisms in high concentrations, it is convenient to wash the diatoms by transferring them into an intermediate bath (drop of nutrient solution on a microscope slide) and to bring them into the culture vessel in the next step.
As only a part of the isolated species is expected to reproduce, it is advantageous to put different species together in a culture vessel.
This first rough culture should be checked after a few days with regard to increase of contained organisms. It is not unlikely to find unwanted organisms that multiply faster than the ones you have been looking for. Single-celled green algae or small species of diatoms (eg Nitzschia) can reproduce in a few days to such an extent that isolation of the desired diatoms becomes difficult. In this situation one has to act quickly and transfer the desired diatoms into a new culture vessel. In the case of heavy contamination, it is necessary to wash the diatoms in intermediate baths. However this is not always successful, because on the often sticky surfaces of diatoms, for example, single-cell green algae adhere very well. It is recommended to set up new cultures in parallel each inoculated with one or a few diatoms.
The video on the left (180x time lapse) shows an example of contamination. It is the first culture from a sample with several Pinnularia contaminated unintentionally with diatoms of the genus Fragilaria and flagellates (time lapse). Examples of contamination with epiphytic diatoms and amoebae can be seen by clicking on the links in this sentence.
If a reproduction occurs of one or more species, the desired candidates are transferred into the next culture dish. Starting a new culture with a single diatom creates a clonal culture. The risk that this culture is not developing well is significantly higher than with cultures that are launched with several diatoms.
Temperature
Our cultivated species are from local Central European waters. They thrive well at room temperature. If one intends to cultivate diatoms from arctic regions, one must keep them at lower temperatures.
Dynamics of growth
Batch cultures of diatoms undergo the typical phases, which are also known from other microorganisms.
After a lag phase without multiplication of the cells, exponential growth follows. When resources (in particular nutrients and carbon dioxide) get limited, cell division slows down and a stationary phase is reached. Here no increase takes place. Finally, death phase (decline phase) starts.
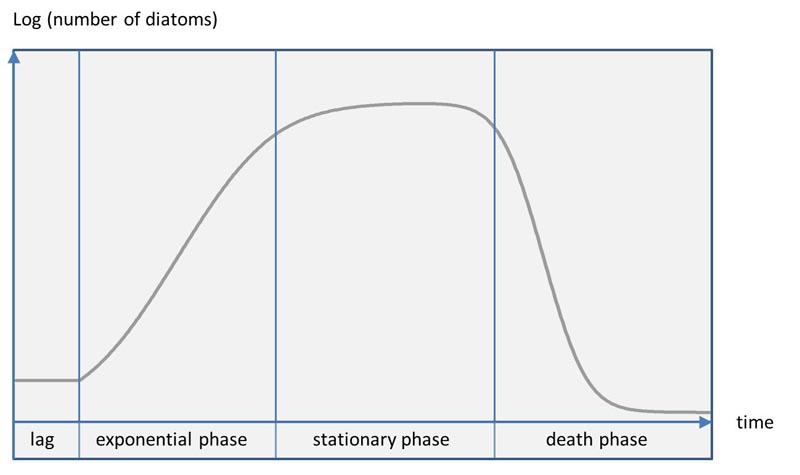
The temporal duration of the phases is strongly dependent on the species and the environmental conditions. While some small Nitzschia species have already undergone the cycle after two weeks, some Rhopalodia or Pinnularia species can be kept successfully in a small petri dish with moderate light for several months. Some diatom species exhibit a stable phase over weeks without striking cell divisions or dying out.
Observation of the cultures
The cultures should be checked regularly. A weekly inspection has proved to be a good thing for me. It is possible to determine whether they are suitable for removing diatoms for observation or "repotting". Negative developments such as sudden death of Diatoms or the prevalence of bacteria are not to be missed.
As soon as one cultivates more than a few cultures, it is important to keep the overview. Culture vessels must be labeled for identification, for example with a sequence number. As a contemporary lab book, one can use a spreadsheet (e.g., MS-Excel) or a database. With each new culture an entry is created, which is maintained and supplemented with every check-up. At the beginning, the following data should be included at least:
- Date on which the culture was prepared
- The genus or species, as far as it is known
- The culture from which it was inoculated, unless it was freshly prepared from a sample
- Information on the nutrient medium used (type, concentration, pH)
- Environmental conditions such as light source and light intensity
For the reviews, the information may be supplemented by:
- Date of the last inspection
- Growth phase (for instance “exponential growth”)
- Rough estimation of the density of diatoms (for instance "well developed")
- Identifiable contaminations with other organisms
- Removal of diatoms for observation, if this is relevant
- Observations, such as the detachment of a biofilm
- When a culture is disposed, it is recommended to enter the corresponding date.
Finally, a few hints are given:
- As not every culture develops successfully, it is recommended to set up cultures in parallel. The medium and environmental conditions may be varied.
- If a species is to be maintained for a long time, it is necessary to transfer live diatoms into a new culture vessel before the death phase. It is advisable not to wait too long, but to remove diatoms when the culture shows a good appearance.
- Culture vessels should be kept closed except for withdrawals. This protects them against contamination.
- Pipettes can be reused if they have been cleaned. It is usually sufficient to rinse them with hot distilled water.