| Cymbella spec. in dark field (40x time lapse) | Visualization of the motion of the diatoms from the video by calculating the maximum over all frames (click to enlarge) |
Movement
A number of benthic species have the ability to move. Nearly all of them have a raphe. On smooth ground they glide in straight or curved paths, the shape of the paths depends on the curvature of the raphe. They also show complex movements such as sudden reversing, turning around the apical axis, erecting, horizontal rotation about one point of the cell, pirouettes in the erected state, etc. Overall, the movements seem to be randomly and it is not necessarily clear what benefits are generated.
Possible benefits are in particular:
- Optimization of light conditions, because many motile species show positive or negative phototaxis. A photophobic response can also be observed in which diatoms react strongly on local changes in the light intensity with reversal of the direction of motion.
- Periodic vertical migration of diatoms inhabiting sand deposits in particular in intertidal zones. These sediments can be disturbed by tides and currents (see review article Harper (1977))
- Search for places with better nutrient concentration or other favorable chemical environmental conditions (chemotaxis). In the publication of Karen Grace V. Bondoc et al (2016) it is shown that Seminavis robusta moves towards a silicate source.
- Colonization of new habitats
- Search and approach to a partner for sexual reproduction. The structure and function of sexual pheromones has been elucidated for Seminavis robusta (Frenkel, Johannes. PhD Thesis (2014) and Bondoc et al. (2016))
- Leaving the copulation envelope in certain species (see post about sexual reproduction)
An approach to the target of the movement achieves a diatom by varying the movement activity, in particular by controlling the duration of the movement in one direction.
At this point I would like to make a comment from my point of view. It is common to all mentioned advantages of the movement that they are due to a change of location. When you look at the movement of some species, you can doubt whether this can always be the motivation. Cymatopleura elliptica usually rotates slowly around a vertical axis, interacts uncontrolled with the environment and comes hardly from the spot (see video). In many cases, the benefit of mobility is only temporary, as in sexual reproduction. Nevertheless, the majority of the diatoms in a culture is independent of their size always in motion. In certain cases, the benefits could also have a physiological background such as the regulation of energy balance.
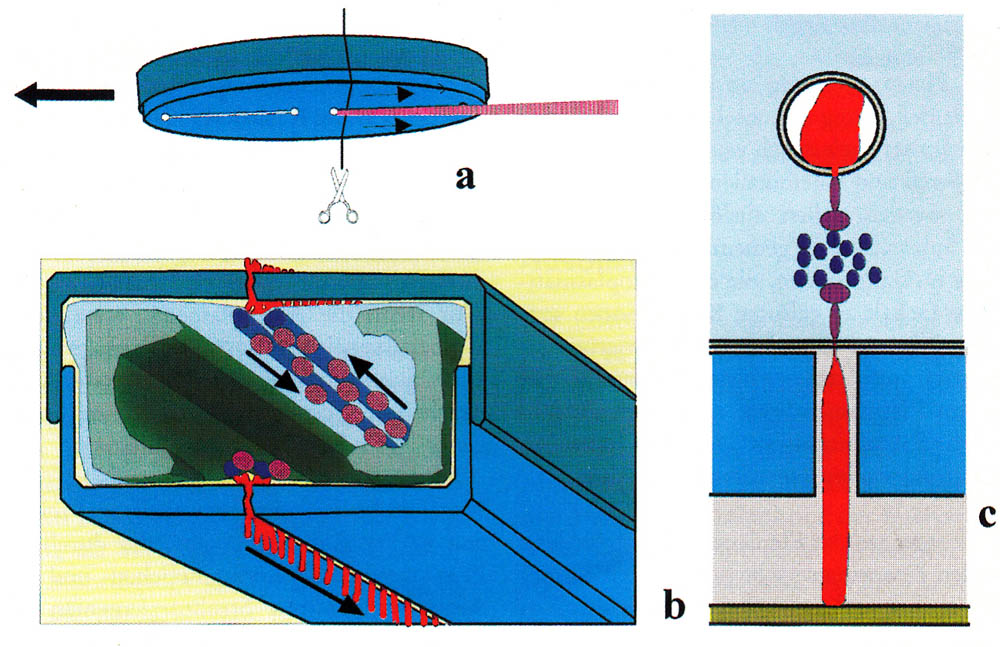
Since the discovery of the movement of diatoms, one struggles for an understanding of the mechanism of motion. As early as 1838, Ehrenberg described a snail-like foot for creeping (Die Infusionsthierchen als vollkommene 0rganismen. 1838 p. 175), which was not proved to be correct. Up to this day, there are numerous theories; none of them is definitively confirmed. They range from a movement due to capillary effect to a cytoplasm flow and to the recoil principle. Today a secretion of mucilage through the raphe and a movement of the mucilage along the raphe are usually used as an explanation, whereby muscle proteins serve as a drive. The prevailing hypothesis is given by Edgar, L.A. & Pickett-Heaps J.D. (1984). A compact representation was created by Menzel, D. and O. Vugrek (1997) and is reproduced below with the original illustration and translated text:
|
|
| Schematic representation of the sliding motion in patented diatoms. (a) Bottom of a migrating cell. A mucilage trail is formed from the posterior raphe. (b) cross-section along the line in (a). The silicified cell wall consists of two parts (blue-gray), which, like the two halves of a petri dish, are on top of each other. The chloroplasts are shown in green; the nucleus in the center of the cell is omitted for the sake of clarity. Underneath the raphe is a pair of actin filaments (blue), which are used as pathways for the transport of mucilage vesicles (pink). The vesicles fuse at the ends of the raphes with the plasma membrane. Each vesicle unloads a mucilage trail (red) to the outer side of the membrane where it swells and is pressed through the gap of the raphe. One end of the thread adheres to the substrate, the other remains bound to the cell membrane, and it is assumed that this end is connected with a motor molecule (dark violet), which transports the thread along the actin bundles. Upper and lower raphe produce mucus filaments at the same time. (c) detail of b. |
| Menzel, D. and O. Vugrek, Muskelproteine in Pflanzenzellen. Biologie in unserer Zeit, 1997. 27(3): p. 195-203. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission. |
Two known problems of this theory are mentioned here:
- The movement of myosin on actin filaments always takes place in one direction. The movement of the diatoms, however, changes the direction of motion, with no difference in speed. Diatoms have one or two raphe systems on one valve. Each raphe system allows movements in both directions. In some species one can even observe that particles are transported in the opposite direction along the same raphe and collide (Nultsch, W. (1962)).
- The raphe of many motile diatoms is not simply an open cleft. Some move in spite of an almost closed tongue in groove slit. Other mobile species do this by means of a canal raphe, which is located on the valves and is connected to the interior of the cell only by a series of pores.
At least amendments are necessary to explain the observations by the described actin-myosin transport.
J. Wang, S. Cao, C. Du and D. Chen have investigated the movement at Navicula sp. and propose an updated model. In this model the diatoms are moved by pseudopods protruding out of the valves. One can not necessarily assume that this model can be applied to other genera as well.
On this homepage, observations will be added to the already existing countless ones on the movement behaviour of diatoms. According to my means, these are always observations with the light microscope and one can ask what is still to be observed at all after 200 years of light microscopic examination. In fact, much is being reproduced. Other observations perhaps encourage the reader to his own observations. In several cases I could not check how far these are described in the literature. I would be grateful for hints. Such observations cannot yield a new explanation of the mechanism of motion but may allow a critical look at the described model conception.
Edgar, L.A. & Pickett-Heaps J.D. (1984), Diatom locomotion., Progress in Phycological Research Vol. 3: 47-88
Frenkel, Johannes. PhD Thesis (2014). Struktur und Funktion von Sexualpheromonen der Diatomee Seminavis robusta. Friedrich-Schiller-Universität Jena, Biologisch-Pharmazeutische Fakultät
Harper, M.A. (1977). Movements. In: The Biology of Diatoms, (D. Werner, ed). 224-249, Blackwell, Oxford
Karen Grace V. Bondoc, Jan Heuschele, Jeroen Gillard, Wim Vyverman & Georg Pohnert. Selective silicate-directed motility in diatoms. Nature Communications http://dx.doi.org/10.1038/ncomms10540
Bondoc, Karen Grace & Lembke, Christine & Vyverman, Wim & Pohnert, Georg. (2016). Searching for a Mate: Pheromone-Directed Movement of the Benthic Diatom Seminavis robusta. Microbial Ecology. 72. 10.1007/s00248-016-0796-7.
See also:
Jeroen Gillard, Johannes Frenkel, Valerie Devos, Koen Sabbe, Carsten Paul, Martin Rempt, Dirk Inzé, Georg Pohnert, Marnik Vuylsteke, Wim Vyverman: Metabolomik unterstützt die Strukturaufklärung eines Sexualpheromons von Kieselalgen., Angewandte Chemie, DOI: 10.1002/ange.201208175
Nultsch, W. (1962) Über das Bewegungsverhalten der Diatomeen., Planta 58: 22.
Wang, J., Cao, S., Du, C. & Chen, D. Underwater locomotion strategy by a benthic penate diatom Navicula sp. Protoplasm 250, 1203–1212 (2013).