|
|
|
|
Eunotia sp. (300x time lapse) and superimposed images |
Eunotia sp. (150x time lapse) and superimposed images |
The movement of Eunotia sp.
As the previous examples have shown, different properties of the paths of diatoms on smooth substrates can be understood on the background of the shape of their raphes. These involve path curvature, position of the center of force and the characteristics of curvature at reversal points. However, trajectories are not always easy to interpret. The movement in Cymatopleura elliptica shows (see video) that certain forms of valves in connection with the shape of the raphe only allow a random movement. This post will address Eunotia. The movement of diatoms of this genus is unusual even in the plane, i.e. without erecting.
Species and Raphe
In March 2018 a Eunotia sp. was isolated in a pond near Stuttgart/Hohenheim (48°42'34.0"N 9°12'29.1"E) and kept in clonal culture. I assume that this is Eunotia glacialis Meister 1912. In view of the existing images, it could also be Eunotia glacialifalsa, Eunotia glacialispinosa, Eunotia belgica or Eunotia valida, according to indications by Dr. Nélida Abarca (Botanic Garden and Botanical Museum Berlin, Free University of Berlin). Corresponding to the documented ranges of the mentioned species in Baden-Württemberg (Mattern et al. 2019) the assignment to
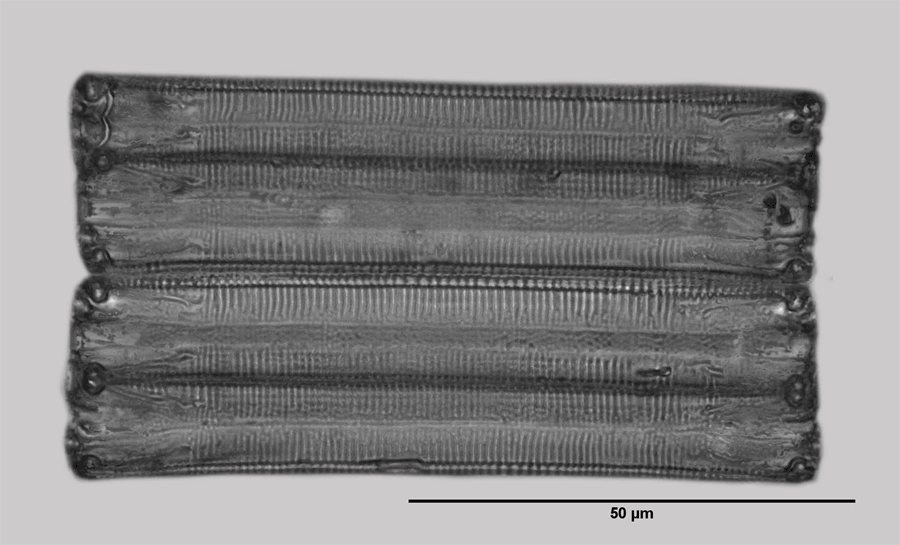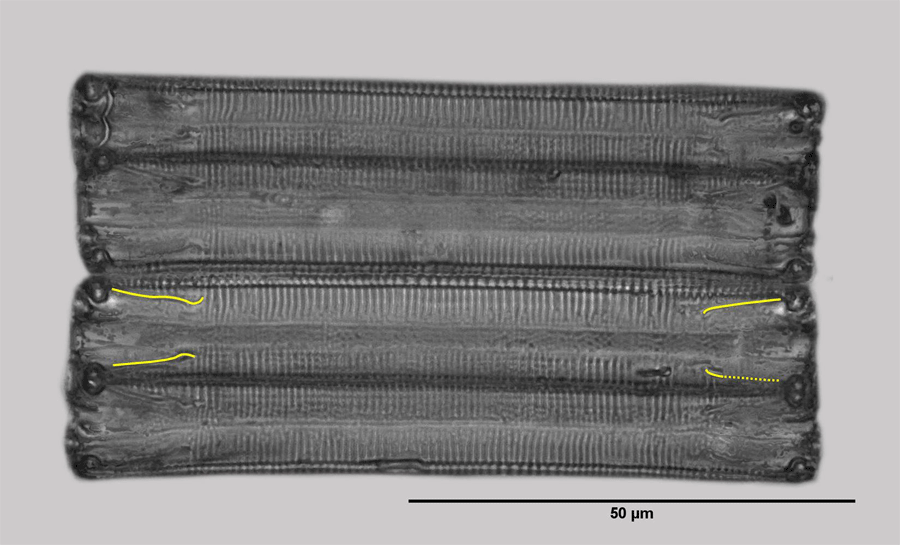
 Eunotia glacialis seems probable. Since a residual doubt remains, it is referred to as Eunotia sp. here. The pictures on the left show the girdle and valve view. The diatoms have just developed from cell division and therefore their width (expansion in the direction of the pervalvarous axis) is small.
Eunotia glacialis seems probable. Since a residual doubt remains, it is referred to as Eunotia sp. here. The pictures on the left show the girdle and valve view. The diatoms have just developed from cell division and therefore their width (expansion in the direction of the pervalvarous axis) is small.
In the girdle view, the short raphe is periodically displayed as a yellow line, as far as it is clearly recognizable. The picture of the valve view suggests that the raphe at the poles still runs a bit on the upper side of the valve. Such raphe branches are typical for Eunotia.
Usually these diatoms lie on the girdle bands on the substrate. Apparently, at least in culture, the concave side usually faces the substrate. In this position, up to four raphe branches can have contact with substrate and contribute to the propulsion. The criteria for the analysis procedures already described are apparently not fulfilled here.
Motility
In the following, only the movement in the plane in girdle position is considered. The movement patterns of Eunotia pectinalis described by Bertrand (1993) impressively show that the raphe systems of this species also enable various movements in which the diatom stands upright. I could not observe such movements. In the observed species in culture, tilting into the valve position occurred only as a result of collisions between diatoms.
The cultivated Eunotia forms chain-like colonies, which can become very long in vitro. Some pictures can be found on the pages on the size sequence and synchronicityronicity of chain-like colonies. The individual diatoms that have separated from colonies are mobile. Only rarely one observes several connected diatoms in motion. This is why they can probably also be colonised epiphytically. In the first raw culture, besides Eunotia, Cymbella was also present, which colonized Eunotia colonies. The video on the left (4x time lapse) shows the situation.
In the two videos at the top of this page (300x and 150x time lapse) you can see some diatoms in motion. Below each of them there is an image gallery with superimposed frames in different time intervals. The second video on the left shows the movement in higher magnification and 20x time lapse. Below it are again different superimpositions.
In time-lapse, the movement appears erratic. The direction of movement and orientation of the cell change frequently. Therefore, the movement of the center of the diatoms was used to measure the velocity. The speed is relatively low. In the measurements carried out, it was between 1.1 µm/s and 2.3 µm/s. With a length of about 70 µ from apex to apex, the diatom typically takes between half a minute and one minute to cover its own cell length.
Forms of movement
The forms of movement are marked by the raphes and their activity. In Eunotia in girdle position, they are versatile, as four independent raphe branches can be in contact with the substrate at the same time. As the videos of movement and the superimposed images show, the apical axis 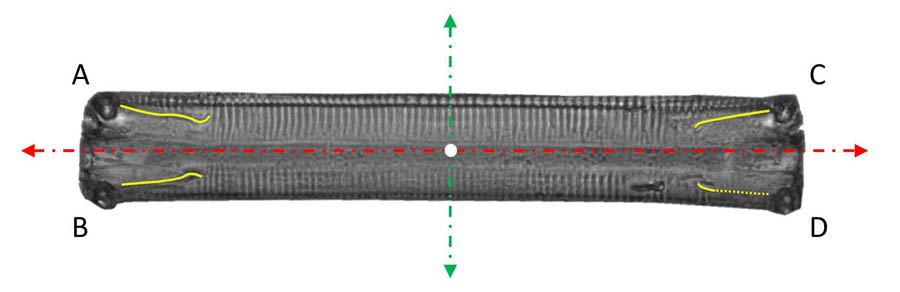 (dashed, red in the picture on the left) and the trajectory of the center (marked white) of the diatom usually form a clearly observable angle, i.e. the alignment of the diatom is rarely tangential to the movement of the center. Besides the velocity component in the direction of the apical axis, there is thus usually an essential component in the direction of the pervalvarous axis. Often the motion occurs roughly in the direction of the diagonals of the rectangular contour line (in direction of theline segments AD or BC). This suggests that opposite raphe branches (e.g. the raphe branches at A and D) contribute mainly to the propulsion and are active in the same direction.
(dashed, red in the picture on the left) and the trajectory of the center (marked white) of the diatom usually form a clearly observable angle, i.e. the alignment of the diatom is rarely tangential to the movement of the center. Besides the velocity component in the direction of the apical axis, there is thus usually an essential component in the direction of the pervalvarous axis. Often the motion occurs roughly in the direction of the diagonals of the rectangular contour line (in direction of theline segments AD or BC). This suggests that opposite raphe branches (e.g. the raphe branches at A and D) contribute mainly to the propulsion and are active in the same direction.
Occasionally the component in the apical direction becomes very small or disappears and the diatom moves in the direction of the pervalvarous axis (dashed, green). This can be explained by an activity of the raphe branches in the same direction, for example at A and C.
Large components of movement in the direction of the pervalvarous axis give the movement of the diatoms in time-lapse photography something floating. They seem to drift, although they have a strong cohesion to the substrate.
Rotation
Rotary movements can also be observed. The following video shows a rotation around a full circle. Below the video you can see superimposed images with the same time interval.
In addition to the high regularity, it is noticeable that the pivot point is slightly outside the diatom. An evident explanation of the rotational movement is that only the two raphe branches A and B (or C and D) are in contact with the substrate and their activity proceeds in opposite directions. One raphe works in apical direction, the other in distal direction. This is sketched in the following picture.

In the specific case, a rotation around 2π took almost exactly 60 seconds, which means an angular velocity of ω = 0.105 radians per second. As the width of the diatom is 24.8 µm, the radius of the circle is approximately r = 12.4 µm. With the relation v=r*ω the velocity at the raphe is 1.26 µm/s. This corresponds to the typical velocity of this species.
Motion sequences
The given description is roughly simplified, because the directions of movement change continuously. Rotations and diagonal linear movements often overlap, so that fan-like structures appear on superposed images. On the other hand, the orientation of the apical axis does not necessarily change in case the trajectory of the center changes direction.
In many motile diatoms one can observe that longer paths are traversed and suddenly a reversal of direction takes place. Here, a raphe branch is usually in contact with the substrate, whose direction of activity changes. This is not visible in a clear manner in the observed Eunotia. Only a jerking back and forth occurs, during which a change of direction is difficult to recognize. A possible explanation is that in Eunotia up to four raphe branches can contribute to movement and it is very likely that more than one raphe branch contributes to movement. Then the reversal of the direction of movement of a raphe branch leads to a change of direction, but not to a reversal of the diatom.
Notice
On the subject of the movement of diatoms of the genus Eunotia Ehrenberg, Paula Furey has published the following paper, which also refers to this page:
Furey, Paula C. "Motility in the Diatom Genus Eunotia Ehrenb." Diatom Gliding Motility (2021): 185-209.
Bertrand, J. (1993) Mouvements des diatomées. III. Le pivotement polaire vertical de Eunotia pectinalis (Kütz) Rab. Essai de quantification des forces. Cryptogam. Algol. 14(4), 157-172.
Mattern, H., Stutz, S., van de Vijver, B. and King, L. (2019). Klasse Bacillariophyceae. In: Beiträge zu den Algen Baden - Württembergs. Band 2: Spezieller Teil: Euglenozoa und Heterkontobionta (Ed. by S. Stutz & H. Mattern), pp. 94‒371. Verlag Manfred Hennecke, Remshalden.